Conference opening + Introductions
09h00 – 09h30
Registration & Welcome
Networking breakfast
Poster exhibition + booths
09h30 – 09h40
Conference opening
Sofie Bracke, Deputy Mayor of Ghent, responsible for Economy, Port and Sports
09h40 – 10h00
Introduction to CESPE & Update building
Prof. Thomas De Beer, CESPE Director
Dr. Christoph Portier, CESPE General Manager
PART 1:
Perspective from the pharmaceutical and biopharmaceutical Industry
– Goals – State-of-the-art – Bottlenecks –
Delegates from the big pharmaceutical and biopharmaceutical companies will highlight their company’s perspective on the current goals, state-of-the-art and bottlenecks related to their big investments in the transition towards sustainable manufacturing.
10h00 – 10h20
Environmental sustainability in the Small Molecules Active Pharmaceutical Ingredient’s
synthesis: from global policy into action.
Bert Heirman, Program Manager Strategic Sustainability Initiatives – Small Molecules, Janssen Pharmaceutica Geel
&
Bie Lambert, Head of Operations for Janssen Pharmaceutica Geel, Janssen Pharmaceutica Geel
10h20 – 10h40
Journey to a net-zero plasma derived manufacturing facility
Simon Gilleman, Sustainability Manager, Takeda
10h40 – 11h00
Coffee break
Poster exhibition + booths
11h00 – 11h20
(Preliminary) Sustainability in the Biomanufacturing Industry:
Global ambitions – Role of the big pharma – No-brainers – Struggles
David Vertongen, Sr. Technical Design Lead, Pfizer
11h20 – 11h40
Sustainability during Drug Product Manufacturing Development.
Jan-Sebastiaan Uyttersprot, PAT Principal Scientist, UCB Pharma
PART 2:
Contributions from Academic & Industrial stakeholders
– Chemical manufacturing – Biotech manufacturing – Drug Product Manufacturing –
Three consecutive sessions will be held where both academic and industrial stakeholders will showcase how their innovative approaches could play a crucial role in the sustainability advancements in different areas within pharmaceutical and biopharmaceutical manufacturing.
Session 1 – Chemical manufacturing
11h40 – 11h55
Digital technologies reducing the footprint in Pharma, examples from the Industry
Dirk Wollaert, Manager Business Development Pharma USA, Siemens Digital Industries
11h55 – 12h10
Transitioning to a more sustainable API development
Prof. Chris Stevens, Principal Investigator, CESPE
12h10 – 12h25
Towards a better process understanding and intensification by modeling
Prof. Joris Thybaut, Principal Investigator, CESPE
12h25 – 13h55
Networking lunch
Poster exhibition + booths
Session 2 – Biotech manufacturing
13h55 – 14h10
How Sustainability Meets Efficiency in Freeze-Drying
Mathieu Massart, Engineering Manager, RheaVita
14h10 – 14h25
Data, Artificial Intelligence & Analytics for Sustainable (Bio)Pharmaceutical Manufacturing.
Noah Nzuki, ESG Lead EMEA, Cognizant
14h25 – 14h40
Pharmaceutical Freeze-Drying: Innovations for a Smaller Ecological Footprint
Carolin Reinke, Senior Innovation Manager Pharma & Healthcare, GEA
Session 3 – Drug Product manufacturing
14h40 – 14h55
SUSTAIN: SUstainability STrAtegy ImplementatioN, from ESG to the working plant
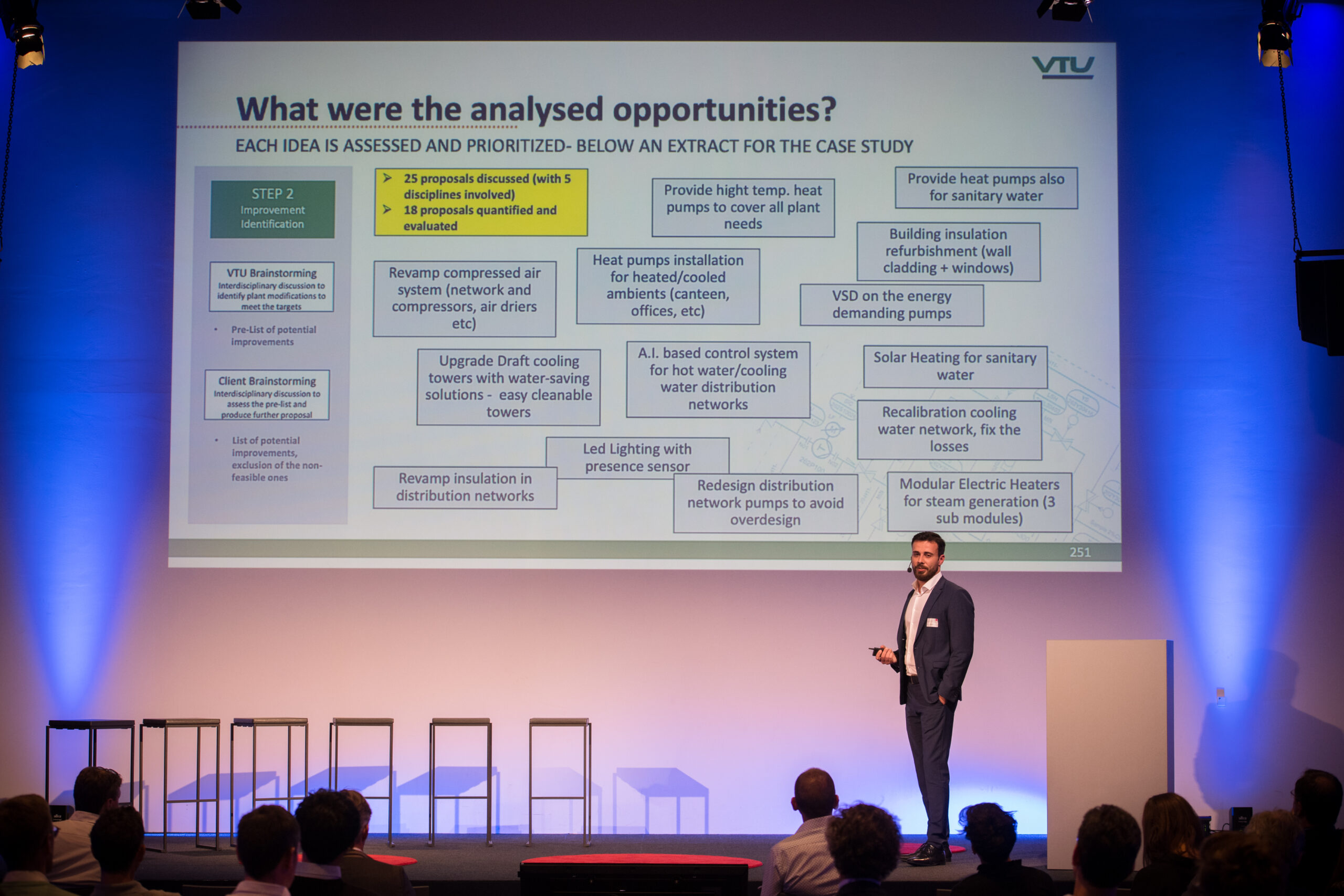
Christian Post, Senior Process Engineer, VTU
14h55 – 15h10
Use of microfluidics to revolutionize the production of pharmaceuticals
Tim Dieryckx, CEO, Voxdale
15h10 – 15h25
The triple bottom line in healthcare: A holistic sustainability assessment for decision support
Prof. Delphine De Smedt, Principal Investigator, CESPE
15h25 – 15h45
Coffee break
Poster exhibition + booths
PART 3:
Staying ahead of what’s next
– Roundtable discussion – Innovation Café –
15h45 – 16h30
Roundtable discussion
Representatives from different key stakeholders groups will have an open discussion about the current challenges and innovations facilitating the transition towards sustainable pharmaceutical and biopharmaceutical manufacturing.
Get inspired by the insights of these key opinion leaders from industry, academia and policy makers.
Moderator
Tineke Van Hooland, Founder and CEO, Epic 10
Panel
Talia Flanagan, Head of Product Design and Performance, UCB Pharma
David Vertongen, Sr. Technical Design Lead, Pfizer
Simon Gilleman, Sustainability Manager, Takeda
Noah Nzuki, ESG Lead EMEA, Cognizant
Delphine De Smedt, Principal Investigator, CESPE
Isabelle Francois, Director Innovation, MEDVIA
16h30 – 17h20
Innovation Café
The talented people within CESPE will have the chance to pitch their innovative research to a broader audience.
Don’t miss this sneak-peek in the ongoing research within the different CESPE groups and member companies!
Take a look at the submitted abstracts for the Innovation Café.
Closing
17h20 – 17h30
Closing word
Prof. Dieter Deforce, Principal Investigator, CESPE
Networking Reception & Dinner
17h30 – …
Poster exhibition + booths